
Expert Consensus: Targeted Solutions for Variant Interference and Quality Control Drift: "Riding the East Wind of Consensus, Breaking Clinical Deadlocks" with the Litop Medical HbA1c Detection Scheme
2025 Year 6 Month 28 Day, the "Expert Consensus on the Clinical Application of Point-of-Care Testing for Glycated Hemoglobin" was published in "Laboratory Medicine and Clinics," 2025 pointing out POCT technology provides a new approach for glycated hemoglobin testing Its simplicity and speed (< ) characteristics improve the efficiency of diabetes management, 10min accelerating the "detection-feedback-intervention" closed loop improving the timeliness and accuracy of management, and the equipment is small and portable It can be extended to community and family settings, which is of great significance to primary-level diabetes prevention and control. Glycated hemoglobin (
) testing is the core of diabetes diagnosis and management, reflecting the average blood glucose over the past HbA1c ~3 2 months, and is a key basis for the diagnosis and treatment plan of diabetes and its complications. However, the accuracy of the test faces challenges
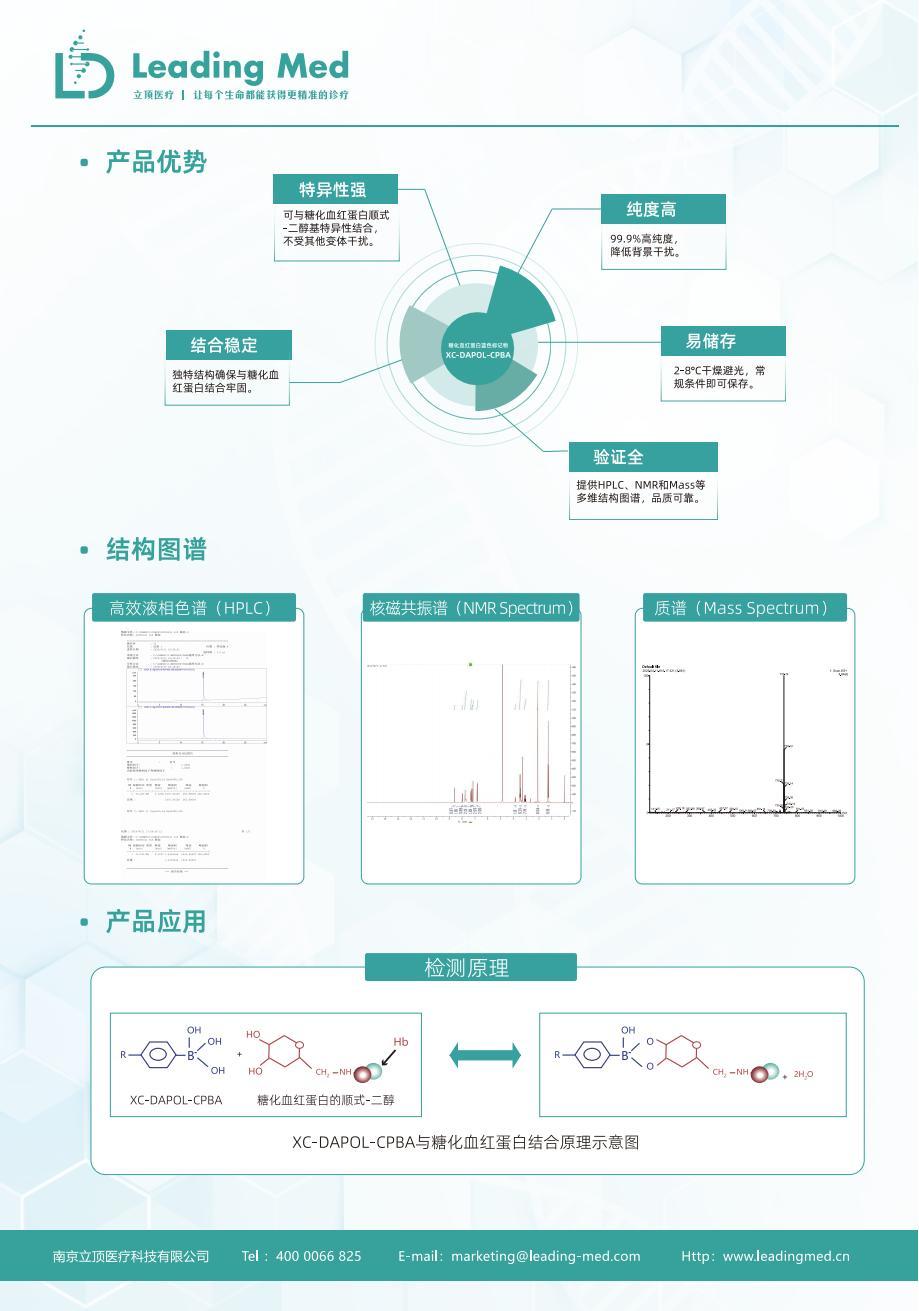
With each increase in error of : 0.5% the risk of misdiagnosis and missed diagnosis increases Hemoglobin variants can lead to detection bias 23% 1.2%-2.5% Insufficient quality control can also lead to result drift [1-4] The relevant expert consensus emphasizes the importance of accurate testing and standardized quality control, while Litop Medical is based on point-of-care testing ( ) technology, boric acid affinity chromatography technology provides a new approach for glycated hemoglobin testing principle launched glycated hemoglobin blue marker ( ) XC-DAPOL-CPBA and glycated hemoglobin quality control product combination scheme, which meets the consensus requirements, breaks through from both specificity and stability dimensions, and provides strong support for precise diagnosis and treatment [5-6] I. Precise breakthrough of variant interference: the targeted recognition mechanism of blue marker 。
The total prevalence of hemoglobin variants in the Chinese population is approximately
0.222% In the south HbE prevalence is approximately 0.58% Traditional detection methods have misjudgment, such as ion exchange HPLC The misjudgment rate is about 34.7% prevalence is approximately Immunoassay for HbS cross-reactivity rate is about 8.3% which may affect the test results [6-7] In comparison, boric acid affinity chromatography can avoid the interference of the above variants, and has the advantages of fast detection and stable results, thus achieving more accurate detection 。
[8] HbA1c In boric acid affinity chromatography chromatography 。
method detection, the blue marker is an important raw material, Litop's glycated hemoglobin blue marker has the characteristics of specific binding to the cis-diol group of glycated hemoglobin, specific color marking, stable structure, no interference from other hemoglobin variants, high purity, easy storage, and comprehensive verification. II. Glycated hemoglobin quality control product: guarding the key link to ensure the accuracy of detection - The use of quality control products is the core of ensuring
accurate detection: technology provides a new approach for glycated hemoglobin testing Real-time monitoring of the precision and accuracy of detection
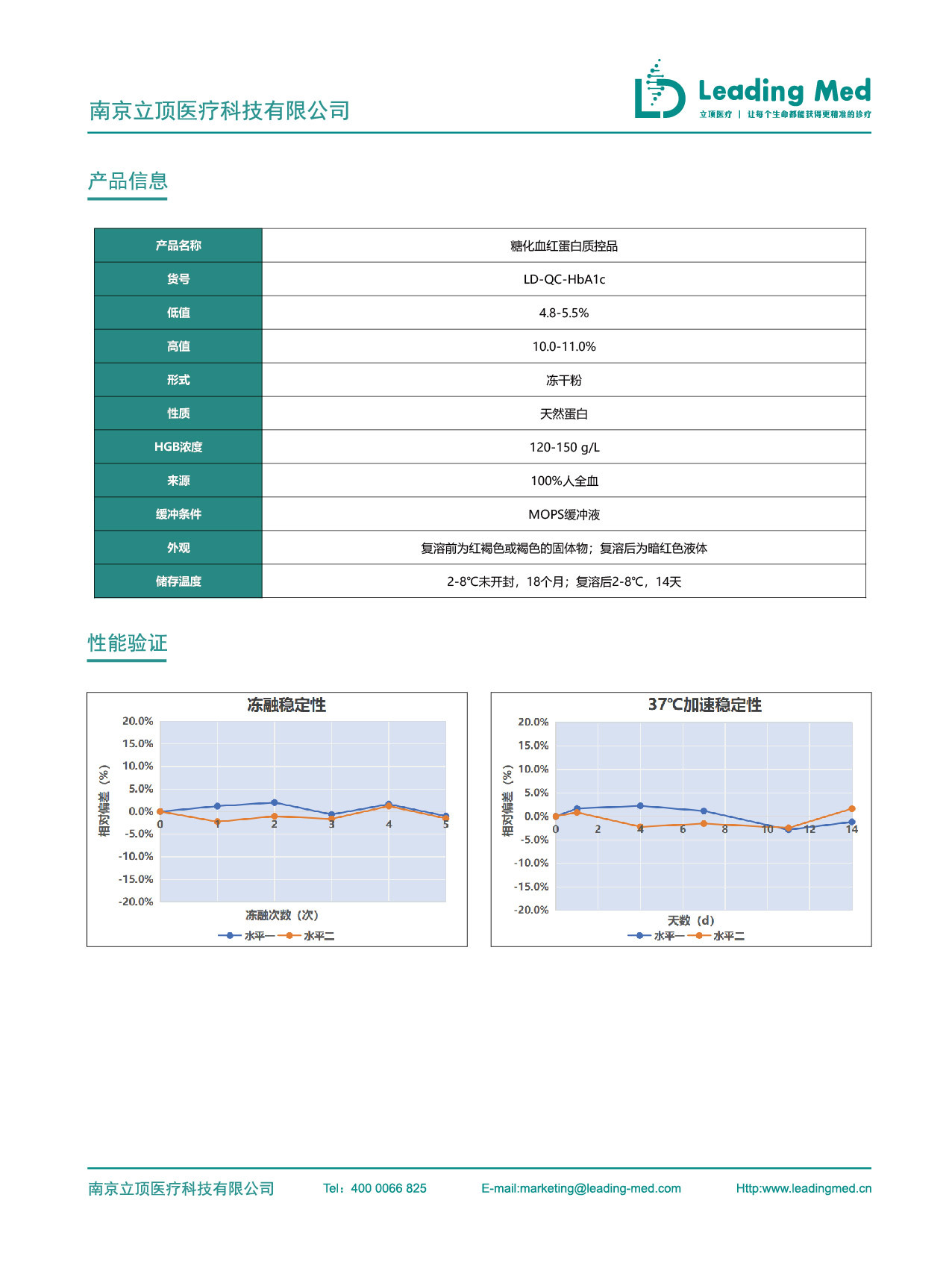
offsetting interference from variants and operations, ensuring that the results are traceable to international standards; technology provides a new approach for glycated hemoglobin testing Supporting clinical decision-making safety and reducing the risk of misdiagnosis is a necessary step to meet the requirements of standardized management and achieve standardized testing [5,9] Therefore, Litop launched derived from healthy human whole blood, close to real clinical samples, with small inter-bottle differences, good uniformity, and strong stability, which can be used for laboratory 。
internal quality control of detection, ensuring the detection results combination scheme, which meets the consensus requirements, breaks through from both specificity and stability dimensions, and provides strong support for precise diagnosis and treatment of 100% accuracy HbA1c and repeatability. References: [1]Chakravarthy, S.N., Ramanathan, S., S, S. et al. EP15A3 Based Precision and Trueness Verification of VITROS HbA1C Immunoassay. Ind J Clin Biochem. 2019, 34: 89–94. 重复性。
参考文献:
[1]Chakravarthy, S.N., Ramanathan, S., S, S. et al. EP15A3 Based Precision and Trueness Verification of VITROS HbA1C Immunoassay. Ind J Clin Biochem. 2019, 34: 89–94.
[2]Standardization of HbA1c. In: IFCC standardization oh HbA1c. National Glycohemoglobin standardization program. 2010.
[3]Rehnuma B, Ibrahim M, Nasir TA. Quality assurance and quality control in clinical laboratories. Pulse. 2015,8: 62–65.
[4]Little RR, Rohlfing C, Sacks DB. The National Glycohemoglobin Standardization Program: Over 20 Years of Improving Hemoglobin A1c Measurement. Clin Chem. 2019, 65(7): 839-848.
[5] Expert consensus on the clinical application of point-of-care testing for glycated hemoglobin 2025.
[6] Zhou Xianghai, et al. Expert consensus on the identification of hemoglobin variants affecting the clinical application of glycated hemoglobin. Chinese Journal of Diabetes, 2023,31 (8):561-570.
[7] Song Beiling , Wu Jiong , Hu Jiahua , etc. . The impact of common hemoglobin variants on different glycated hemoglobin detection systems [J]. Inspection Medicine ,2024,39(12):1219-1223.
chromatography Liu Zhiwei , Zhang Guojun , Hou Lixian , etc. . Evaluation of the effect of boric acid affinity chromatography for the detection of glycated hemoglobin A1c的效果评估 [J]. Chinese Journal of Tropical Medicine , 2010,(1):2.
[9] Yan Bin . Quality control of point-of-care testing for glycated hemoglobin . Modern Journal of Laboratory Medicine ,2007,22(4):109-110.
2022-07-26










 xy13584019024
xy13584019024