
Ningpu, Jiangsu launches molecular pathology diagnostic FFPE quality control product for tuberculosis
2024-11-15
Tuberculosis is a serious infectious disease that seriously endangers human health, "2023 Global Tuberculosis Report" [1] shows that in 2022, the number of new tuberculosis cases worldwide reached 10.6 million, a new historical peak. As one of the high-burden countries for tuberculosis, China has a large population of tuberculosis patients. In 2022, the number of new tuberculosis cases accounted for 7.1% of the global total, ranking third among the 30 high-burden countries. [1] However, in 2021, the etiological diagnosis rate of tuberculosis in China was only 58%, lower than the global average diagnosis rate (63%). [2] Pathological diagnosis is the most important tuberculosis diagnosis method besides microbiology, playing an irreplaceable role in the diagnosis and differential diagnosis of tuberculosis. [3] 。
The "T/CRHA 029-2023 Tuberculosis Pathology Diagnosis Standard" group standard (hereinafter referred to as the "Standard") issued by the China Research Hospital Association's Supermicro and Molecular Pathology Professional Committee on December 25, 2023, [4] points out: Positive MTB molecular pathology detection is the most important step in confirming tuberculosis , emphasizing the important position of molecular pathology detection technology in tuberculosis diagnosis.
Comparison of Tuberculosis Diagnostic Methodologies
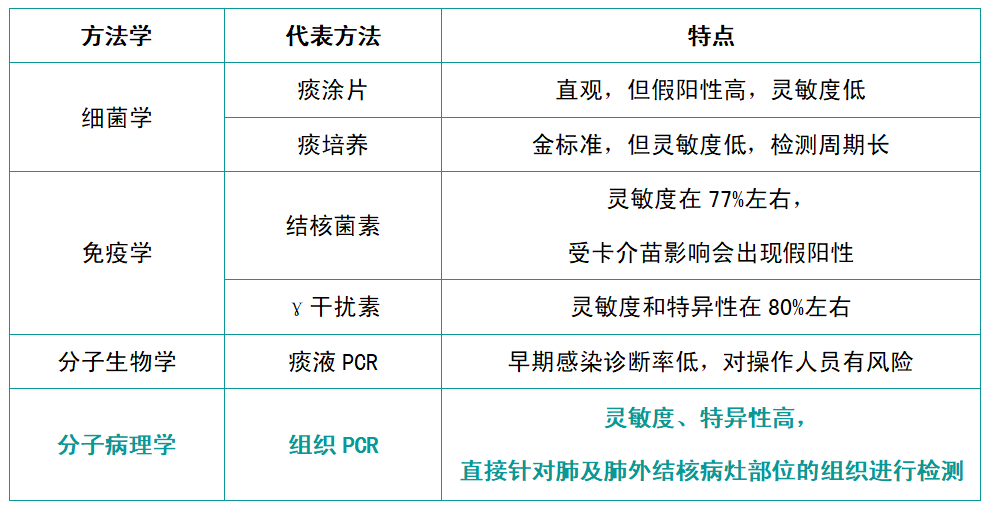
Currently, for this tuberculosis molecular pathology diagnostic product, Jiangsu Ningpu uses the specific sequence IS6110S of the Mycobacterium tuberculosis complex to establish a special paraffin-embedded tissue (FFPE) quality control product, filling the gap in the market and improving the reliability of such detection.
Product Features:
3 positive quality control products + 2 negative quality control products, one wax block can cut 300 FFPE sections
Use dPCR to detect the number of IS6110 copies in FFPE samples to confirm its sensitivity and specificity
Derived from cell lines with complete traceable documents to ensure traceability of quality control products
Usage Process

References:
[1]World Health Organization. Global tuberculosis report 2023. Geneva:World Health Organization, 2023.
[2]World Health Organization. Global tuberculosis report 2022. Geneva:World Health Organization, 2022.
[3]Chinese Medical Association Tuberculosis Branch, Expert Consensus Writing Group on Tuberculosis Pathology Diagnosis. Expert consensus on tuberculosis pathology diagnosis in China. Chinese Journal of Tuberculosis and Respiratory Diseases, 2017,40(6):419-425. doi: 10.3760/cma.j.issn.1001-0939.2017.06.005.
[4]China Research Hospital Association. T/CRHA 029-2023 Tuberculosis Pathology Diagnosis Standard. 2023-12-25.

